To avoid the cross contamination, the endoscope ought to be disinfected after using. But how to accurately evaluate the disinfection efficacy, it has been a difficult problem for a long time.
TAILIN innovatively developed the endoscope microbial limit test system to solve this problem.
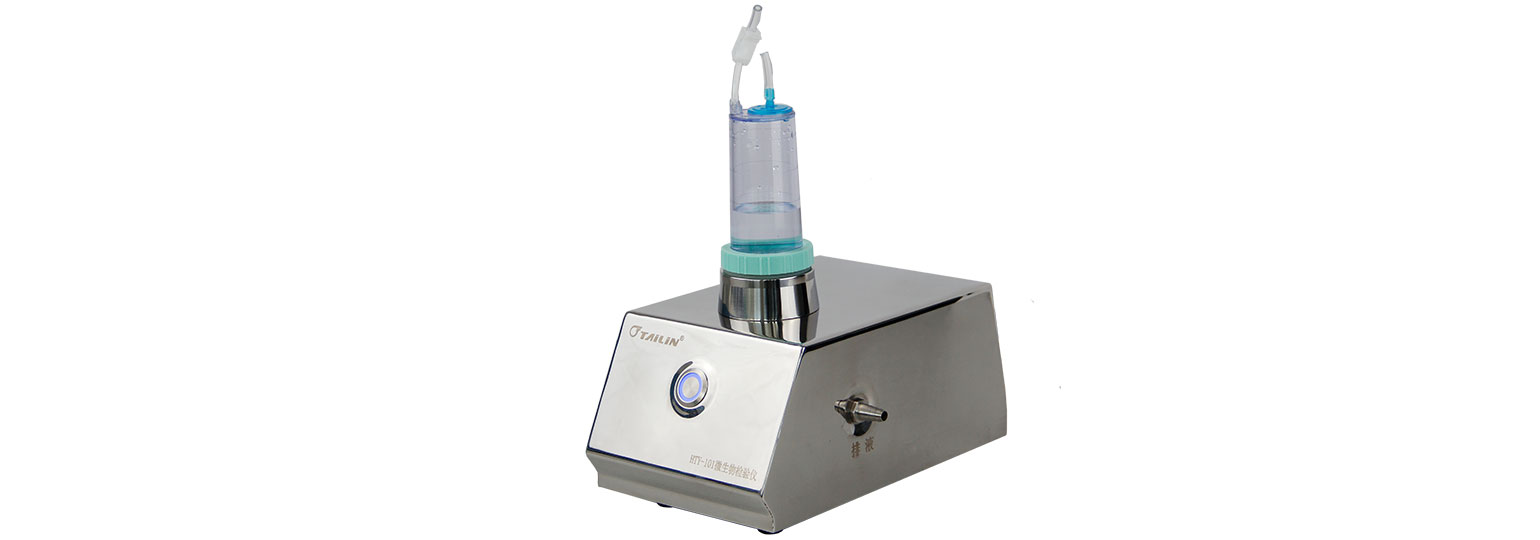
The Endoscope Microbial Test System consists of HTY-WP02 peristaltic pump for sampling,HTY-101 diaphragm pump for suction filtering, and FC501 filtration cup.
Step 1: Rinse the endoscope with sterile water, and transferring the rinsed water in FC501 filtration cup by HTY-WP02 peristaltic pump;
Step 2: Install the FC501 on the HTY-101 diaphragm pump;
Step 3: Start the HTY-101 diaphragm pump, the sampling rinsed water will filtering through the FC501 filtration cup.
Step 4: The microorganism will be hold on the filtration membrane, if there is any.
Step 5: Put the filtration membrane in the culture media for incubation and counting.
The Endoscope Microbial System can fit for most of endoscope brands; it is closed system which can avoid the external contamination when checking the endoscope disinfection/cleaning efficacy.
Closed membrane filtration method;
Built-in diaphragm pump for efficient filtration;
Small size, less space occupation;
On/Off button with indicator light, simple and intuitive;
Concise internal pipeline without dead corner inside;
Made of polished mirror stainless steel, easy to clean and disinfect.
Power supply: AC 220V/50Hz
Power consumption: 25W
Pumping flow: ≥1200mL/min
Noise: ≤60dB (load condition)
Net Weight: 2.5kgs
Dimensions: 15cm x 25cm x 11cm
Drainage tube: inner diameter Φ7mm~Φ11mm silicone
Consumables: FC501 filtration cup
Used for the sampling during endoscope microbial test.
Technical specifications are subject to change without notice. Copyright reserved by Tailin.


 CN
CN